Furosemide 8mg Syrup
COMPOSITION of Furosemide 8mg Syrup
Each ml contains:
Furosemide 8 mg
Flavoured syrupy base q.s.
DESCRIPTION
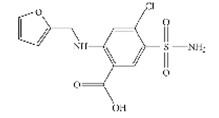
Furosemide Syrup 8mg/mL Chemical Structure
DOSE AND METHOD OF ADMINISTRATION of Furosemide 8mg Syrup
Furosemide Syrup Oral Administration
Oedema
Therapy should be individualised according to patient’s response. This therapy should be titrated to gain maximal therapeutic response with the minimum dose possible to maintain that diuretic response.
Adults:
The usual initial daily dose is 20 to 80 mg given as a single dose. If the diuretic response to a single dose of 20 to 80 mg is not satisfactory, increase this dose by increments of 20 to 40 mg, not sooner than 6 to 8 hours after the previous dose, until the desired diuretic effect is obtained. This individually determined dose should be given once or twice (e.g. at 8 am and 2 pm) daily. The dose of Furosemide may be carefully titrated up to 400 mg/day (except in advanced renal failure) in those patients with severe clinical oedematous states. The mobilisation of oedema may be most efficiently and safely accomplished by giving Lasix on 2 to 4 consecutive days each week.
When doses exceeding 80 mg/day are given for prolonged periods, careful clinical laboratory observations are particularly advisable.
Infants and Children:
The usual initial dose of oral Lasix for infants and children is 2mg/kg body weight given as a single dose. If the diuretic response is not satisfactory, the dose may be increased by 1 to 2 mg/kg no sooner than 6 to 8 hours after the previous dose. Doses of greater than 6 mg/kg body weight are not recommended.
For maintenance therapy in infants and children, the dose should be adjusted to the minimum effective level.
Hypertension
Therapy should be individualised according to the patient’s response. This therapy should be titrated to gain maximal therapeutic response with the minimum dose possible to maintain that therapeutic response.
Adults:
The usual initial daily dose of Lasix for hypertension is 80 mg, usually divided into 40 mg twice a day. Dosage should then be adjusted according to the response. If a response is not satisfactory, add other antihypertensive agents. Changes in blood pressure must be carefully monitored when Lasix is used with other antihypertensive drugs, especially during initial therapy.
To prevent an excessive drop in blood pressure, the dosage of other agents should be reduced by at least 50% when Lasix is added to the regimen. As the blood pressure falls under the potentiating effect of Lasix, a further reduction in dosage or even discontinuation of other antihypertensive drugs may be necessary.
SPECIAL WARNINGS AND PRECAUTIONS FOR USE Furosemide 8mg Syrup
Excessive diuresis may result in dehydration and reduction in blood volume with circulatory collapse and with the possibility of vascular thrombosis and embolism, particularly in elderly patients.
Excessive loss of potassium in patients receiving cardiac glycosides may precipitate digitalis toxicity.
In patients with hepatic cirrhosis and ascites, initiation of therapy with Lasix is best carried out in hospital. Sudden alterations of fluid and electrolyte balance in patients with cirrhosis may precipitate hepatic coma, therefore, strict observation is necessary during the period of diuresis.
Cases of reversible or irreversible tinnitus or hearing impairment have been reported. Usually, reports indicate that Lasix ototoxicity is associated with rapid injection or infusion, severe renal impairment, hypoproteinaemia, doses exceeding several times the usual recommended dose, or concomitant therapy with aminoglycoside antibiotics, ethacrynic acid, or other ototoxic drugs. In patients with hypoproteinaemia, e.g. associated with nephrotic syndrome, the effect of Lasix may be weakened and its ototoxicity potentiated.
Cautious dose titration is required. If the physician elects to use high dose parenteral therapy, controlled intravenous infusion is advisable (for adults with normal renal function, an infusion rate not exceeding 4 mg Lasix per minute must be used; for adults with impaired renal function [creatinine > 5 mg/dL], an infusion rate of no greater than 2.5 mg per minute must be used). Caution should be exercised when administering curare or its derivatives to patients undergoing furosemide (frusemide) therapy. It is also advisable to discontinue furosemide (frusemide) for one week prior to any elective surgery. Caution should be exercised and the risks and benefits of combining risperidone with Lasix or other potent diuretics should be considered prior to the decision to treat. In the risperidone placebo-controlled trials in elderly patients with dementia, a higher incidence of mortality was observed in patients treated with furosemide (frusemide) plus risperidone (7.3% ; mean age 89 years, range 75 to 97) compared to treatment with risperidone alone (3.1% ; mean age 84 years, range 70 to 96) or furosemide (frusemide) alone (4.1% ; mean age 80 years, range 67 to 90).
Concomitant use of risperidone with other diuretics (mainly thiazide diuretics used in low doses) was not associated with similar mortality findings. No pathophysiological mechanism has been identified to explain this finding and no consistent pattern for cause of death was observed.
Nevertheless, caution is advised. Irrespective of treatment, dehydration was an overall risk factor for mortality and should, therefore, be carefully avoided in elderly patients with dementia. Rigid sodium restriction is conducive to both hyponatraemia and hypokalaemia, thus strict restriction of sodium intake is not advisable in patients receiving furosemide (frusemide).
Furosemide (Frusemide) should be used with care, especially in the initial stages, in patients with impairment of micturition (e.g. prostatic hypertrophy). Urinary outflow must be secured. In patients with a partial obstruction of urinary outflow (e.g. in patients with bladder-emptying disorders, prostatic hyperplasia or narrowing of the urethra), increased production of urine may provoke or aggravate complaints. Thus, these patients require careful monitoring.
SHELF LIFE
24 months from the date of manufacture. Once opened 8 weeks.
STORAGE
Stored below 25°C. Protect from light.
Marketed by:
PYXUS PHARMACEUTICALS PVT. LTD.


